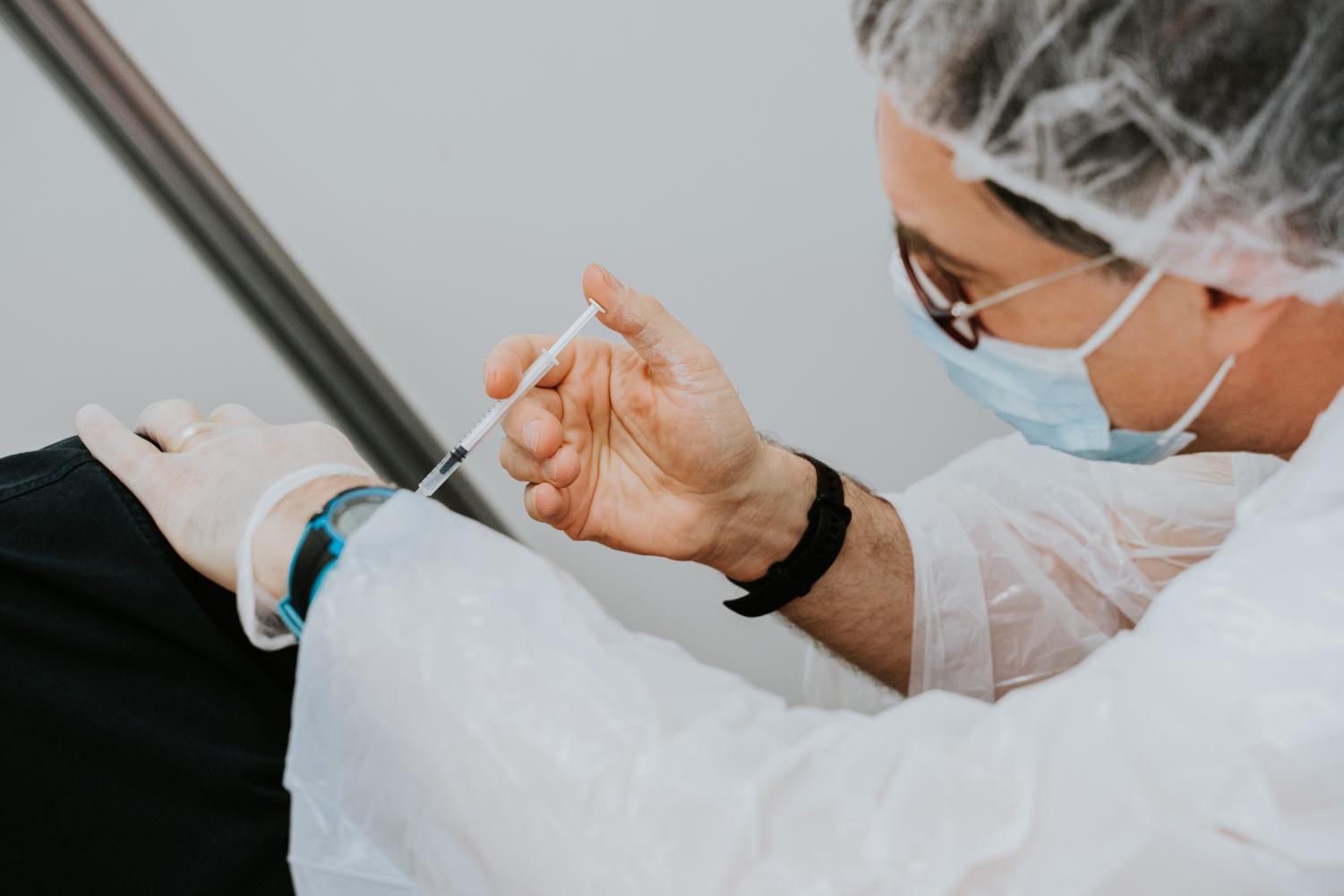
The U.S. will see nearly a 90% drop in Johnson & Johnson vaccine doses nationwide, according to an article by The Washington Post. Due to manufacturing issues, the government has cut down J&J’s vaccine allotment to only 700,000 doses this week, showing a concerning decrease from last week’s near 5 million administered doses.
A DECLINE OF DOSES
ABC News reports that last week California received more than 574,000 doses of the J&J vaccine. This week, the state is only receiving 12% of that amount. Next week, the number will decrease further to only 4%.
“There are roughly 18 million people living in the state that have yet to receive a shot, according to estimates from 2020 census data. But, the state’s current supply is around five million doses,” said Stephanie Sierra from ABC News.
This comes at a critical time since vaccine eligibility will be expanded to all Californians 16 and older by Thursday, April 15.
Now, public health officials are pondering whether this could impact the state’s plan to reopen the economy in June. Chief Pharmacy Executive for University of California San Francisco Health Desi Kotis expects to have the answer by May. Kotis explained that there should be large quantities of Pfizer and Moderna vaccines coming to California, which have accounted for most of the nation’s vaccination efforts.
15 MILLION DOSES QUARANTINED
The New York Times reported that workers at a coronavirus vaccine manufacturing plant in Baltimore combined ingredients from the J&J and AstraZeneca vaccines. This error caused 15 million doses to be quarantined and have delayed the rest of the shipments.
The Baltimore plant is overseen by Emergent BioSolutions, a manufacturing partner to both J&J and AstraZeneca—a vaccine that has yet to be approved by the Food and Drug Administration for authorization in the U.S.
J&J delivered a statement late Wednesday to address the steps they are taking with Emergent to hold fast on their statement made earlier this year to deliver 24 million doses by the end of April. This depends on whether or not they satisfy FDA regulators for future doses.